
Lower energy configurations are more stable, so things are naturally drawn toward them. Throughout nature, things that are high in energy tend to move toward lower energy states. Deviations from this ratio result in charged particles called ions. This one-to-one ratio of charges is not, however, the most common state for many elements.

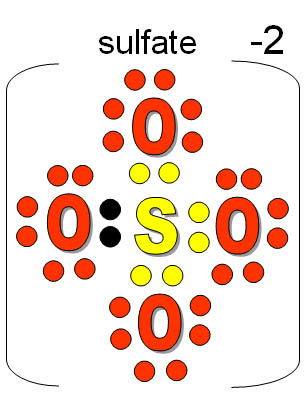
The overall charge on the atom is zero, because the magnitude of the negative charge is the same as the magnitude of the positive charge. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. Hence, the hydroxide ion has nine protons and ten electrons.4.1 Introduction to the Octet Rule 4.2 Ions and the Periodic Table Common Cations Common Anions Ions of Transition Metals 4.3 Ionic Bonding 4.4 Practice Writing Correct Ionic Formulas 4.5 Naming Ions and Ionic Compounds 4.6 Polyatomic Ions 4.7 Naming Polyatomic Ions 4.8 Properties and Types of Ionic Compounds 4.9 Arrhenius Acids and Bases 4.10 Ions, Neurons, and Action Potentials Also, hydroxide has a net -1 charge and we know there must be an extra electron compared to the number of protons. In a neutral molecule, the number of protons and electrons are equal. Total protons=protons in H + protons in O How many protons and electrons are in a hydroxide ion?Īns: We can calculate the total number of protons in a hydroxide ion by adding up the number of protons in one hydrogen atom and one oxygen atom: However, other polyatomic ions form with the -3 charge, but the borate and phosphate ions are the ones to memorize. Polyatomic ions with a -2 charge are also common. Bisulfate (or hydrogen sulfate) – HSO 4 –.Bicarbonate (or hydrogen carbonate) – HCO 3 –.Many of the polyatomic ions have an electrical charge of -1. The electron from the NH 4 transfers to the OH creating an OH – ion and an NH 4 + ion. It needs two electrons but has only one from the OH hydrogen atom. When ammonium forms an ionic bond with an OH the extra electron transfers to complete the outermost shell of the OH. Then four electrons are available from the hydrogen or one more than needed. When it shares electrons covalently with four hydrogen atoms. Nitrogen has five electrons in its outermost shell, and it can have eight. Ammonium is one of the few positively charged polyatomic ions and doesn’t contain oxygen. In most polyatomic ions, it contains oxygen and a negatively charged anions. The sulfate receives the two electrons to become SO 4 -2. In sulfuric acid, the sulfate forms ionic bonds with the hydrogen atoms that donate an electron each to become hydrogen ions, H+. The 4 oxygen atoms would need to have eight electrons shared between them, leaving a deficit of two. It shares them covalently with the oxygen atoms having six electrons in their outer shells. The sulfur atom has six electrons in its outer shell. For example, sulfuric acid H 2SO 4 contains H + and the polyatomic SO 4 -2. Many common chemicals are polyatomic compounds with polyatomic ions.

Therefore compared to the neutral atom, they have extra electrons for negatively charged anion or not enough electrons for the positively charged cation. The ion has a net charge over it because the total number of electrons is not balanced by the total number of protons in the nucleus. Monatomic ions are the atoms which are ionized by gaining or losing electrons. Polyatomic ions can be compared with monatomic ions. NO 3 − :: Nitrate ClO − :: Hypochlorite Structure of Polyatomic Ions NO 2 − :: Nitrite HCO 3 − :: Hydrogen carbonate NH 4 + :: Ammonium, CO 3 2− :: Carbonate This is because there are two or more atoms present in the polyatomic ion. Whereas they behave externally like monatomic ions, but their internal structure is more complicated. They easily dissolve in water and conduct electricity and dissociate in a solution similar to other ions. In addition, polyatomic ions and those ionic compounds take part in chemical reactions such as acid-base, precipitation, and displacement similar to monatomic metallic ions. Compounds formed from such a combination of ions are polyatomic ionic compounds.īut the polyatomic ion will behave as a single unit. Polyatomic ions are covalently bonded groups of atoms and having a positive or negative charge caused by the formation of an ionic bond with another ion. This article will give details of polyatomic ions and their examples. Examples of monatomic ions include Na+ and Cl- etc. Thus it is different from monatomic ions, which contain only one atom. The term poly means many, so a polyatomic ion is an ion that contains more than one atom. 1.5 Solved Question for You Polyatomic Ions


 0 kommentar(er)
0 kommentar(er)
